Trial participants receive either standard care, or at least one of the treatments under investigation. Researchers use routine patient data to assess whether the treatments have an impact on the overall number of patients who survive Covid-19, as well as other outcomes such as the length of hospital stay.
Why did this work happen?
When the coronavirus pandemic began, there were no specific treatments available for people severely affected by Covid-19. There was an urgent need for information about whether existing or new drug therapies were effective against the disease.
Normally, a large clinical trial would take many months to set up, but RECOVERY was launched in just nine days and recruited over 10,000 patients across the UK within two months. As of October 2023, the trial has taken place at 190 NHS hospital sites across the UK, with over 48,000 participant recruited. It is open to all patients hospitalised with Covid-19.
How was data used?
When a patient joins the trial, the local research team completes a simple form with crucial information such as which treatments the patient is receiving (for example, if they’re being given oxygen).
With the patient’s consent, the routine data team then links each recruited patient with their record in the database held by the central NHS data custodian – NHSE for England, the SAIL Databank for Wales, Public Health Scotland and the National Records of Scotland. Linkage with data from other organisations such as the UK Renal Registry and the Intensive Care National Audit and Research Centre adds additional information. There are technical and operational safeguards in place to protect data used in the trial. They include encryption and password protection, limiting the number of people who have access to the database and using unique reference numbers to identify participants rather than names wherever possible.
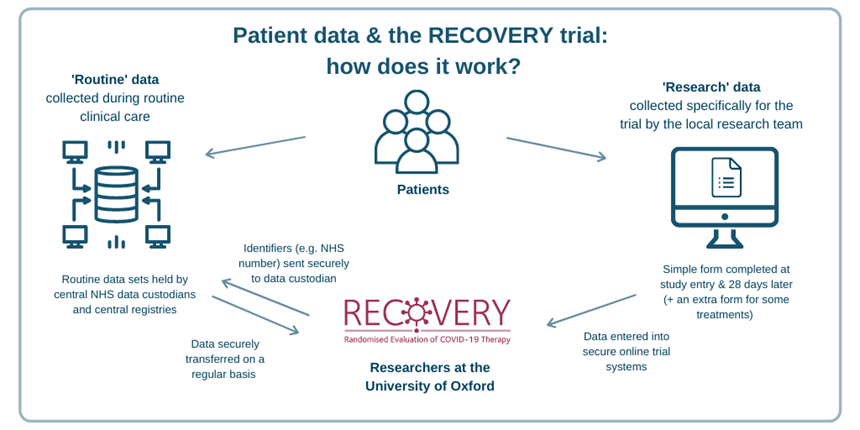
The data controller for the trial is the University of Oxford. The legal basis for processing data is an article in the General Data Protection Regulations which says data processing is necessary if it is in the public interest, which includes national and international public health issues like pandemics.
What were the benefits?
Within just a few months, the trial led to the discovery that the steroid dexamethasone reduces death in ventilated patients by a third. The steroid is now being used to treat people hospitalised with Covid-19 in the NHS and internationally. The trial has also found that four treatments (lopinavir-ritonavir, hydroxychloroquine, azithromycin and convalescent plasma) delivered no clinical benefit, allowing healthcare providers to focus resources away from these. The trial is continuing to investigate several other treatments.
Who funded and collaborated on this work?
The RECOVERY trial is conducted by the registered clinical trials units with the Nuffield Department of Population Health in partnership with the Nuffield Department of Medicine, both at the University of Oxford. The trial is supported by a grant to the University of Oxford from UK Research and Innovation/National Institute for Health Research (NIHR) and by core funding provided by NIHR Oxford Biomedical Research Centre, Wellcome, the Bill and Melinda Gates Foundation, the Foreign, Commonwealth & Development Office, Health Data Research UK, the Medical Research Council Population Health Research Unit, and NIHR Clinical Trials Unit Support Funding.
The trial involves many thousands of doctors, nurses, pharmacists, and research administrators at 177 hospitals across the whole of the UK, supported by staff at the NIHR Clinical Research Network, NHS DigiTrials, Public Health England, Department of Health & Social Care, the Intensive Care National Audit & Research Centre, Public Health Scotland, the Secure Anonymised Information Linkage at the University of Swansea, and the NHS in England, Scotland, Wales and Northern Ireland.
Following the success of the trial in the UK, there is also an international trial being developed, including Ghana, India, Indonesia, Nepal, South Africa and Vietnam.
Where can I go for more information?
- Page updated: 30 May 2024
- Print page


