Why is this work needed?
Some kidney diseases are so rare that conducting research on them is very difficult. Hospitals may only see a few cases of these conditions and so conducting research into why they occur and how they can be treated may be impossible. For conditions like this, it is vital that doctors are able to share examples and expertise to guide their decisions. Researchers also need to have as much data as possible to support their search for new treatments.
What is happening?
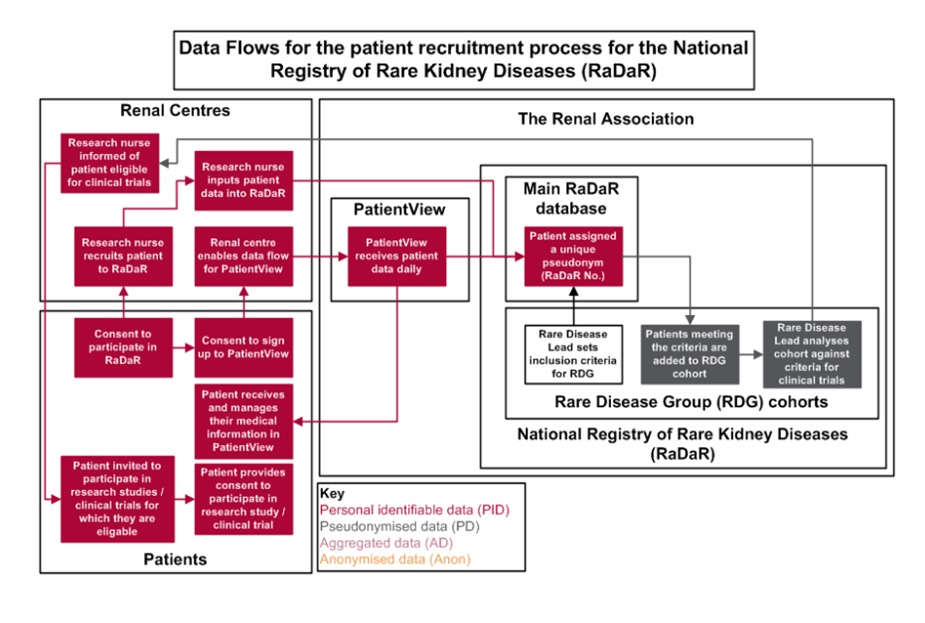
RaDaR is a registry of data on rare kidney conditions, covering multiple different treatment centres across the UK. To date, RaDaR has recruited over 30,000 patients and is the largest rare kidney disease registry in the world. It is now in its thirteenth year and collections information about 30 different rare kidney diseases. Patients can access their own clinical data online via the Patient View website, while researchers can use the platform to collaborate and share information with colleagues and also to recruit people to clinical trials.
What are the benefits?
RaDaR supports research into a range of rare kidney diseases. For example, membranoproliferative glomerulonephritis Type 2 (MPGN) is a rare condition which tends to affect very young children, causing the body’s immune system to attack the kidneys, slowly destroying them. Research using data from RaDaR is exploring possible new treatments that could significantly slow the damage caused to the kidneys of people with MPGN.
Furthermore, by enabling patients to access their own records more easily, RaDaR also supports patients to take a more proactive role in managing their own health data.
What type of data is involved?
Patients who contribute to the registry agree that information such as demographics, blood and urine results, medications, transplant and dialysis history, genetics and comorbidities medications will be entered into the RaDaR database by their hospital research team or kidney unit. Personal data such as name, date of birth and NHS number is pseudonymized so the patient cannot be identified.
Patients are also able to see certain data in Patient View (PV), an online system that records renal patients’ results, medications and clinic letters. RaDaR patients are given a secure log-in to access their own data via PV.
The registry continues to ensure that collected data is as in-depth and up-to-date as possible, such as being able to add historic data about kidney function results, allowing some participants to have results dating back to the 1980’s.
What is the legal basis for accessing the data?
Patients are asked for their consent to participate before their information is entered into the registry. For researchers, patient level data is not released outside of the Renal Association, and instead data analysis is conducted internally before results are shared with the researchers.
Who is funding and collaborating on this work?
RaDaR is supported by a variety of kidney charities including Kidney Care UK, Kidney Research UK, Polycystic Kidney Disease, and the UK Kidney Association, as well as support from NHS England.
Where can I go for more information?
RaDaR Patient Information Sheet
UK Kidney Association – Data & Permissions for RADAR
Renal Interventions – article about RaDaR
- Page updated: 12 December 2023
- Print page


